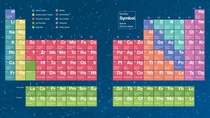
118elements have been described.
Priopćenja i novosti
Ordering the world
The United Nations has declared 2019 the International Year of the Periodic Table of Chemical Elements. It was 150 years ago that Dmitri Mendeleev documented the natural law of chemistry with his fundamental order of the elements – with far-reaching consequences to this day.
It is said anecdotally that the idea came to him in a dream. What is fact is that, 150 years ago, Dmitri Ivanovich Mendeleev brought order to the 63 chemical elements known at that time. The Russian chemist presented the periodic table on October 28, 1869. It sorted the chemical elements into a table in ascending order by the number of protons – positively charged subatomic particles – in their nucleus. The number of protons simultaneously also became the atomic number.
The periodic table is a chart that makes the world and the properties of its elements easier to understand. As a rule of thumb, the elements in each column of the table display similar properties. So far, 118 elements have been described. Most were discovered in the 19th century. Only 10 natural elements were still unknown at the beginning of the 20th century. Today, the main new entries are radioactive elements that do not normally occur naturally but are the result of artificially created nuclear fusion processes. The last four new entries to date came in 2016 with nihonium, moscovium, tennessine and oganesson (numbers 113, 115, 117 and 118).
Despite this, however, the foundations of the world are still far from complete. Scientists are in the process of discovering new, super-heavy elements, using particle accelerators that propel nuclei into each other so that they may fuse to form a new nucleus. This is intended to open up a new, eighth row in the periodic table. Researchers from Japan and the United States announced at the Symposium on Super-Heavy Elements in Poland at the end of 2017 that they were starting the search for elements number 119 and 120. They hope to have found them by 2022.
Mendelevium
In 1955, Dmitri Mendeleev also got his name into the periodic table. When scientists at the University of California in Berkeley, USA, first artificially created the transuranium element, they decided to name the radioactive element mendelevium in his honor. However, its halflife, at just 51.5 days, is only a fraction of the lifespan of the 150-year-old periodic table.